

The MAL Protein, an Integral Component of Specialized Membranes, in Normal Cells and Cancer.
Armando Rubio-Ramos, Leticia Labat-de-Hoz, Isabel Correas, Miguel A.International Journal of Molecular Sciences 2021, 22 Myelin Defects in Niemann–Pick Type C Disease: Mechanisms and Possible Therapeutic Perspectives. Antonietta Bernardo, Chiara De Nuccio, Sergio Visentin, Alberto Martire, Luisa Minghetti, Patrizia Popoli, Antonella Ferrante.A basic model for cell cholesterol homeostasis. Biochimica et Biophysica Acta (BBA) - Biomembranes 2022, 1864

Using cyclodextrin-induced lipid substitution to study membrane lipid and ordered membrane domain (raft) function in cells. Giant plasma membrane vesicles to study plasma membrane structure and dynamics. Cellular and Molecular Life Sciences 2022, 79 MALL, a membrane-tetra-spanning proteolipid overexpressed in cancer, is present in membraneless nuclear biomolecular condensates. Armando Rubio-Ramos, Miguel Bernabé-Rubio, Leticia Labat-de-Hoz, Javier Casares-Arias, Leonor Kremer, Isabel Correas, Miguel A.Human myelin proteolipid protein structure and lipid bilayer stacking. Salla Ruskamo, Arne Raasakka, Jan Skov Pedersen, Anne Martel, Karel Škubník, Tamim Darwish, Lionel Porcar, Petri Kursula.Nature Reviews Molecular Cell Biology 2022, 305 Regulation of membrane protein structure and function by their lipid nano-environment. Central role of Prominin-1 in lipid rafts during liver regeneration. Myeong-Suk Bahn, Dong-Min Yu, Myoungwoo Lee, Sung-Je Jo, Ji-Won Lee, Ho-Chul Kim, Hyun Lee, Hong Lim Kim, Arum Kim, Jeong-Ho Hong, Jun Seok Kim, Seung-Hoi Koo, Jae-Seon Lee, Young-Gyu Ko.The Journal of Physical Chemistry B 2020, 124 Computational and Experimental Advances in Biomembranes: Resolving Their Complexity. This article is cited by 15 publications.
MULTIPASS TRANSMEMBRANE PROTEIN DRIVERS
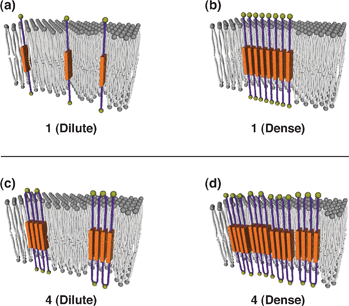
These observations suggest broad conclusions about the composition of ordered membrane domains in cells and point to previously unrecognized drivers of raft affinity for multipass transmembrane proteins. We characterized the potential mechanisms for their exceptional raft affinity and observed that PLP requires cholesterol and sphingolipids for optimal association with ordered membrane domains and that PLP and MAL appear to compete for cholesterol-mediated raft affinity. The only exceptions were two myelin-associated four-pass TMPs, myelin and lymphocyte protein (MAL), and proteo lipid protein (PLP). Across a set of 24 structurally and functionally diverse multipass TMPs, the large majority (92%) had minimal raft affinity. Here we used cell-derived giant plasma membrane vesicles (GPMVs) to systematically measure multipass TMP partitioning to ordered membrane domains. Such determinants have been described for lipids and single-spanning transmembrane proteins however, how multipass transmembrane proteins (TMPs) partition between ordered and disordered phases has not been widely explored. As protein selectivity underlies all functions of lipid rafts, there has been significant interest in understanding the structural and molecular determinants of raft affinity. The hierarchical construction of a higher-order structure by self-assembly of foldamers can be a rational design of programmable functional molecular organisms.Eukaryotic membranes can be partitioned into lipid-driven membrane microdomains called lipid rafts, which function to sort lipids and proteins in the plane of the membrane. Moreover, the tetra block amphiphile shows ion transportation through the membrane following Eisenman sequence XI, where the four molecules self-assemble into a dynamic ion channel with a milli second scale opening-closing motion. The spectroscopic analyses revealed that the tetra block amphiphile, bearing four hydrophobic parts, forms intramolecular stacking of the aromatic portions within the membrane, indicating the formation of an MTM structure. The hydrophobic parts that penetrate the membrane consist of a fluorescent aromatic group, so that absorption and fluorescent spectroscopy allows the characterization of the assembling/disassembling states of the hydrophobic parts. The amphiphiles are composed of alternative hydrophobic and hydrophilic parts. Multiblock amphiphiles adopting a multipass transmembrane (MTM) structure on a bilayer membrane have been developed by mimicking the molecular structures of MTM proteins.


 0 kommentar(er)
0 kommentar(er)
